A GMP inspection uncovers the usual suspects: procedures not followed, validations missing, documentation incomplete. Yet, your team has been through training. The SOPs exist. The systems are in place. So why are the outcomes the same?
This isn’t a systems problem—it’s a thinking problem.
In too many firms, training is treated as a compliance formality rather than a tool of operational transformation. The result? Data piles up. Observations repeat. And teams remain frustrated—checking boxes instead of changing behaviors.
A Background Story
During an engagement at a firm facing ongoing FDA observations, the executive team sat down with me—visibly frustrated, clearly exhausted. The CEO asked a question I hear far too often:
“Why do our people go through all this training but still fail to do what they’re supposed to do?”
My answer:
“Interesting question — and it points to the root issue. To implement true behavioral change, we have to understand how people learn… and how quickly they forget. We also have to understand why they don’t do what they are supposed to do.”
The Astonishing Statistics on Forgetting
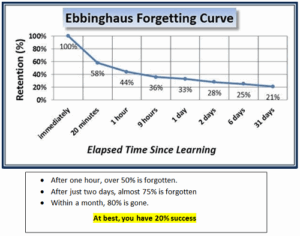
The Ebbinghaus Forgetting Curve shows that within just 24 to 48 hours, most people forget up to 75% of what they learn in a standard classroom session.
If you want to test this:
- Who conducted your GMP training last year?
- What was the topic?
- What changed because of it?
If you’re struggling to answer, you’re not alone. Most annual GMP training is a “check-the-box” event—designed to show auditors that something was done, but rarely designed to ensure something was retained or applied.
So when we gather observation data and chart it by root cause, frequency, or department, the question must follow:
Are we just compiling data to make neat charts?
Or are we prepared to do something that actually changes behavior?
Why Don’t They Do It?
Let’s be honest—most employees know they’re supposed to follow procedures, document actions, validate processes. These are not mysterious concepts.
So why don’t they do it?
The book “Why Employees Don’t Do What They’re Supposed to Do” by Ferdinand Fournies offers powerful insights. Let’s connect them to GMP work environments:
Common Cognitive and Cultural Barriers:
- They don’t understand why it matters.
GMP becomes ritual, not purpose. If you want people to care, tie GMP to patient safety and personal accountability. - They don’t actually know how to do it the right way.
They heard it once. They forgot. Or they never really learned it through real-world application. - They think their way is better.
Shortcuts get reinforced by speed metrics or peer approval. People develop their own “workarounds” that drift from compliance. - There’s no consequence—positive or negative.
If someone cuts corners and nothing happens, they learn it’s okay. If doing it right takes longer and they get scolded for being slow, they learn to cheat the system. - Old habits never changed.
Classic training doesn’t rewrite habits. It informs, maybe. But transformation takes repetition, reinforcement, and leadership modeling.
Training That Works
If you want GMP training to stick, it has to be designed for habit change, not just knowledge transfer.
Principles of Effective Training:
- Break training into digestible, role-specific segments.
Don’t lump everyone into the same room with the same slide deck. - Use storytelling and real-world case studies.
Abstract regulation means little. A story about a failed inspection that mirrors your environment is remembered—and repeated. - Reinforce key concepts multiple times, in different formats.
Repetition builds memory. Application builds behavior. - Involve leadership—visibly and vocally.
Culture starts at the top. If your directors aren’t reinforcing GMP principles in their daily expectations, no amount of training will matter. - Train on the “why” and the “what if.”
Don’t just train the SOP. Train what happens if it’s skipped, ignored, or misunderstood. The consequences are real—so show them.
So, What Should You Do With the Data?
If your trend charts show repeat observations—especially after training—you’re not seeing a knowledge problem. You’re seeing a thinking problem, a habits problem, and a leadership gap.
Don’t just send people back to another training session. Ask:
- Are we making GMP real for our people?
- Are we reinforcing the right behavior on the floor?
- Are supervisors modeling and coaching compliance, or just expecting it?
Final Thought (From Troy’s Perspective)
“Training isn’t about covering content. It’s about shaping culture. If you want GMP compliance that survives longer than a PowerPoint slide, you have to change how people think, what they believe is important, and how they act under pressure.”
Need to Rethink Your Training Strategy?
Compliance Insight offers:
- Behavior-based GMP workshops
- “Three Sins of GMP” leadership sessions
- Observation data interpretation + response planning
- Customized supervisor training for sustainable culture change
Reach out. Let’s stop checking boxes—and start changing behaviors.
