Reach the Horizon
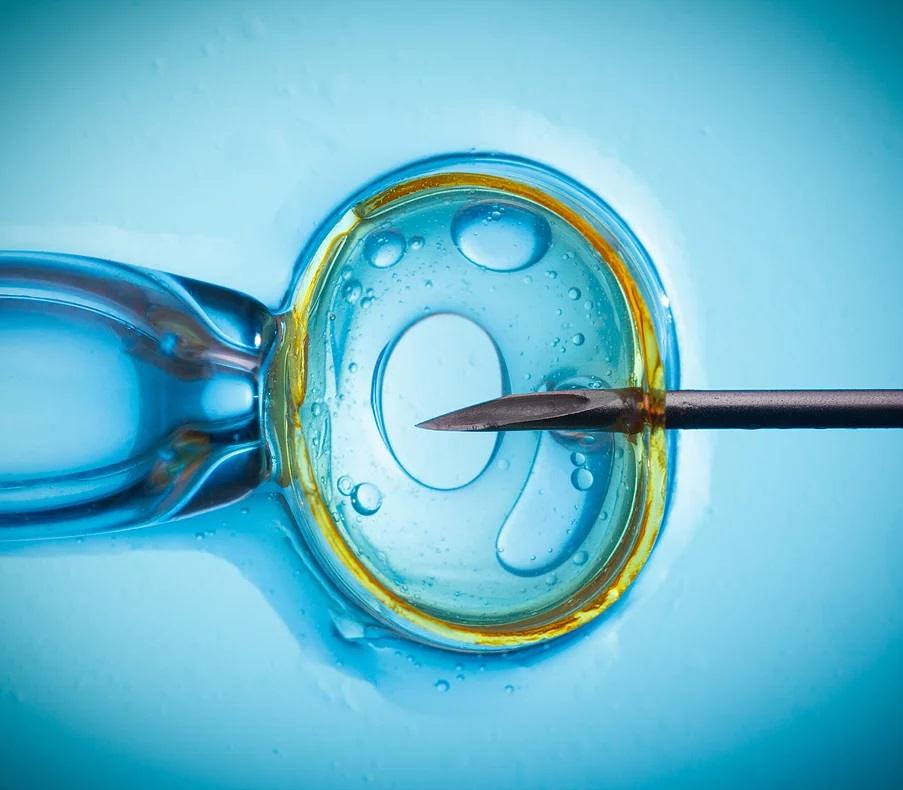
The path to FDA compliance for biologic products is filled with complex regulatory requirements not common in traditional pharmaceutical product development. To get you across the finish line, Compliance Insight offers a full spectrum of Biologics FDA consulting services and provides hands-on assistance through every stage of the FDA regulatory process.
We’ll support you from the early R&D phases of your work on through the entire lifecycle of your Biologics products path to the market. Whatever the nature of your project may be, Compliance Insight possesses the tools and expertise to make your project a success.
Select Services & Capabilities
Quality Management
Clinical Trial Design and Evaluation
GMP Compliance
FDA cGMP Compliance
Regulatory Applications & Submissions
Medical Device Biocompatibility
Regulatory Strategy
FDA and Advisory Meetings
Author, Review and Publish Applications and Amendments
GLP and GCP
Pharmacology/Toxicology
Immunogenicity Assessment
U.S. Agent Services
Auditing & Verification
Market Authorizations
Adverse Event Reporting
