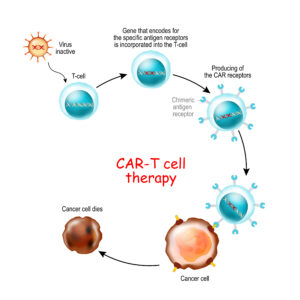
CAR T-cell therapy is a immunotherapy that uses genetically engineered T cells to intensify the immune systems response to cancer. T-cells with Chimeric antigen receptor recognize and kill the cancer cells in the body.
CAR T-cell Therapy
CAR T-cell therapy (Chimeric Antigen Receptor T-cell therapy) is a type of immunotherapy that uses a patient’s own immune cells to target and kill cancer cells. It is a personalized treatment that involves genetically engineering a patient’s T cells (a type of white blood cell) to recognize and attack cancer cells.
The process of CAR T-cell therapy involves several steps:
- Collection of T cells: T cells are collected from the patient’s blood through a process called leukapheresis. This is a similar process to donating blood, where the blood is drawn from one arm and passed through a machine that separates the T cells from the rest of the blood components.
- Genetic engineering: The collected T cells are then sent to a laboratory, where they are genetically engineered to express a chimeric antigen receptor (CAR) on their surface. The CAR is designed to recognize a specific antigen (a protein found on the surface of cancer cells) and trigger the T cell to attack the cancer cell.
- Expansion of CAR T cells: The genetically engineered T cells are then multiplied in the laboratory, creating a large number of CAR T cells.
- Infusion of CAR T cells: The expanded CAR T cells are infused back into the patient’s bloodstream. Once in the body, the CAR T cells seek out and attack the cancer cells that express the targeted antigen.
CAR T-cell therapy has been approved by the FDA for the treatment of certain types of blood cancers, such as leukemia and lymphoma. However, it is still a relatively new and complex treatment with potential side effects, including cytokine release syndrome (CRS) and neurotoxicity. Patients receiving CAR T-cell therapy require careful monitoring and management by healthcare professionals.
To learn more about Car T-cell therapy click here
