Since the beginning of the pandemic, developers have been full-speed ahead creating and receiving approval to distribute Covid-19 tests. To date, there are three types of tests approved for Covid-19 diagnosis: Molecular, Antigen, and Antibody.
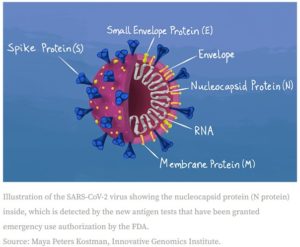
Molecular tests can also be called polymerase chain reaction (PCR), RT-PCR, nucleic acid amplification test (NAAT), or LAMP test. Polymerase chain reaction is used for several laboratory procedures: in this case, detection of bacteria or viruses. PCR is completed in three major steps that are repeated until there is enough material for testing. The steps completed once the sample, primers, Taq polymerase, and nucleotides are assembled in a tube are:
- Heat the double-stranded DNA to a temperature at which it will denature or separate into two pieces of single-stranded DNA.
- Anneal, or cool, the reaction so the primers can bind to their complementary sequences on the newly separated single-stranded DNA.
- Raise the temperature slightly so Taq polymerase can initiate the DNA replication.
This is repeated until 30-40 new strands of DNA are available for sequencing, visualization by gel electrophoresis, etc. Sequencing used in Covid-19 testing to provide positive or negative results, as well as identifying genetic mutations or variants.
Molecular tests are very accurate but are much more expensive than antigen tests. They can be ordered as a rapid result test if the sample location has the appropriate laboratory capabilities but typically take at least a few days to receive results based on laboratory bandwidth.
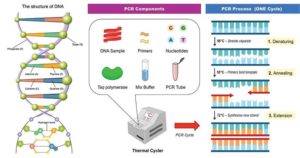
“In immunology, an antigen (Ag) is a molecule or molecular structure, such as may be present on the outside of a pathogen, that can be bound by an antigen-specific antibody or B-cell antigen receptor. The presence of antigens in the body normally triggers an immune response. The Ag abbreviation stands for an antibody generator.” Antigen testing targets these specific proteins on the surface of a virus. There are several methods of performing antigen testing, but each method requires:
- patient samples – In the case of Covid-19, samples are collected via nasal swab or gathering saliva.
- reagent – When exposed to reagent, “viral particles in the sample are disrupted, exposing internal viral nucleoproteins.”
- test devices – Typically reactive strips containing one line of anti-SARS coronavirus monoclonal antibodies and one line of material that reacts with the reagent as a control. For reference, consider an at-home pregnancy test result.
Antigen testing can be completed in as little as 30 minutes, which makes them excellent options for rapid testing and receiving the earliest positive test results; think within 5-7 days of onset symptoms or suspected exposure. It is unlikely to receive a false positive test result, but they are susceptible to false negatives because they are not as sensitive as PCR/molecular tests. If your care provider suspects infection despite a negative antigen test, it is advised to get a follow up molecular test.
Antibody testing differs from molecular and antigen testing, in that it is not used for diagnostic purposes with active Covid-19 infection and instead targets antibodies developed from overcoming the infection. “An antibody is a protein component of the immune system that circulates in the blood, recognizes foreign substances like bacteria and viruses, and neutralizes them. After exposure to a foreign substance, called an antigen, antibodies continue to circulate in the blood, providing protection against future exposures to that antigen.”
The Covid-19 antibody tests target Immunoglobulin M (IgM), the first antibody formed within 1-2 weeks, and Immunoglobulin G (IgG), the second antibody formed, at least two weeks after the illness starts. “IgM will disappear from the body within a few months, but IgG can last for years.” The lifespan of Covid-19 IgG antibodies is still being studied to determine the immunity timeline for those past infection and/or those who have been vaccinated.
Opposed to molecular and antigen tests, antibody tests typically require a whole blood sample. Depending on the test protocol, a traditional whole blood sample may be required or, in the case of Nirmidas Biotech’s recently FDA approved test, a finger prick. This test will greatly change CLIA-waived, point-of-care (POC) settings (doctor’s offices, urgent care, ERs, pharmacies, nursing homes, testing sites, etc.) because it is rapid and tested onsite within 15-20 minutes. Not only is this test rapid, it is also 100% accurate when detecting IgG after 14 days of symptom onset and IgM after 7 days of symptom onset.
There are two methods of antibody testing. The first is laboratory-based immunoassay “which require a reader or analyzer to detect the reaction.” This protocol can determine the amount of the given antibody per unit volume of serum. While this is an effective and accurate tool for measurement, it is costly and takes more time.
The second method of antibody testing is cassette-based systems “which can be read at the [POC] through a change in color in an indicator region visible to the naked eye.” This device has a shallow well in which blood and a small amount of buffer are placed and then absorbed by the porous test strip within the cassette. This test strip has been “impregnated with recombinant viral antigens doped with an indicator;” simply speaking, the indicator reacts with present antibodies and produces a colored line on the test strip to indicate a positive result (again, imagine an at-home pregnancy test result). The Covid-19 test indicates both IgG and IgM with separate lines, giving a loose timeline of infection.
All three tests are constantly being improved upon as we learn more about SARS-CoV-2 and how it’s adapting over time.
| Molecular | Antigen | Antibody | |
| Tests for: | Active infection and/or contagious (active virus “shedding”) | Active infection and/or contagious (active virus “shedding”) | Past infection; not contagious |
| Sample type: | Nasal or throat swab or saliva sample | Nasal or throat swab or saliva sample | Blood sample |
| Rapid vs standard: | Rapid or standard. Can be run while you wait or can be sent to a lab for testing | Rapid or standard. Can be run while you wait or can be sent to a lab for testing | Standard: Sent to a lab for testing. Rapid: finger prick, tested onsite |
| Positive results: | Positive results very accurate | Positive results very accurate | Positive results very accurate |
| Negative results: | Negative result could mean that patient was tested too early or too late | Negative result could mean that patient was tested too early or too late | Negative result could mean that patient was tested too early and had not developed IgM (1-2 wks) or IgG (2+ wks) |
| Cost: | Most expensive | Less expensive | Less expensive |
| Immunity: | No | No | Yes. Scientists are not yet aware of the lifespan of Covid-19 antibodies, specifically IgG. Typically, they can remain in the body for months or years depending on the contagion |
| Helpful: | Determining active infection. Identifying contagious persons | Determining active infection. Identifying contagious persons. Rapid results. Less expensive option | Identify those who had the infection in the past (even asymptomatic). Could create a timeline of infection (based on presence of IgM, IgG, or both. Identify persons that qualify for donation of convalescent blood for the treatment of those currently affected by the virus. Can be used to determine the percentage of the population once infected if enough people are tested |
| Not helpful: | Past infections or developing infections | Past infections or developing infections | Potential negative test if tested too early |
https://dshs.state.tx.us/coronavirus/docs/COVID19-TestingExplained.pdf
https://en.wikipedia.org/wiki/Antigen
https://www.genome.gov/about-genomics/fact-sheets/Polymerase-Chain-Reaction-Fact-Sheet
https://www.fda.gov/media/137885/download
https://asm.org/Articles/2020/August/How-the-SARS-CoV-2-EUA-Antigen-Tests-Work
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7184973/
